Wastewater & Chemical Recycling Publications
Papers
Closed loop recycling systems are an excellent, total solution for recycling wastewater and chemicals used in industrial cleaning processes. Our technical papers explain how closed loop recycling systems can improve cleaning processes, minimize potential regulatory issues, and reduce costs.
 (PDF) "Clean Break from CFCs"
(PDF) "Clean Break from CFCs"
 (PDF) "Encouraging Water and Energy Conservation: Driving Forces"
(PDF) "Encouraging Water and Energy Conservation: Driving Forces"
 "Minimizing Wastewater from Cleaning Processes"
"Minimizing Wastewater from Cleaning Processes"
Books
John Russo extensively updated his original chapter, "Making Decisions about Water and Wastewater for Aqueous Operations," for the Handbook for Critical Cleaning, Second Edition, B. Kanegsberg and E. Kanegsberg, editors, CRC Press, © 2011. This chapter is in Volume One, Cleaning Agents and Systems.
Minimizing Wastewater from Cleaning Processes
By John F. Russo
One recent and prevalent trend has been an increase in the number and stringency of federal, state and local regulations concerning waste generated by reusing the cleaning chemicals and reducing and/or eliminating wastewater discharge.
The best wastewater treatment system should have great adaptability, low environmental impact, low amount of hazardous waste, low environmental impact, low capital outlay and low operating costs.
The objective is to choose a chemical cleaner and equipment that cleans the parts while having the lowest operating cost for site-specific regulations such as air emissions, wastewater and solid waste. The type and amount of cleaning chemicals used will affect both wastewater (cleaning chemical and rinse wastewater) and solid waste.
To select the optimum cleaning process, the amount of wastewater and solid waste generated should be considered as much as the cleaning equipment and cleaning chemistry themselves. No optimum cleaning process can be achieved with certainty unless cleaning chemical, cleaning equipment and wastewater treatment are considered simultaneously - the two major sources or wastewater contamination are the cleaning chemical and the rinse wastewater.
Some solvent and aqueous cleaning chemicals can be reused numerous times. However, unless the chemical volume is reduced and hauled or discharged, disposal can be expensive. For aqueous cleaning chemicals, membrane filtration, such as microfiltration or ultrafiltration, can be implemented to reuse the chemical. Membrane filtration reduces wastewater generation by reusing the wastewater several times. Microfiltration membranes have a coarser rating of about 0.1 to 1 micrometer absolute, while ultrafiltration membranes are finer and rated at about 1,000 to 1,000,000 molecular weight.
What to Choose
Choosing the best method of wastewater reduction depends on numerous factors, such as the key chemical ingredients' molecular weight and characteristics of the filtered contaminants. Testing on the actual cleaning process is one of the better ways to determine the number of reuse cycles. Initial laboratory testing of the cleaning chemical - with or without the contamination - can give an initial indication of membrane compatibility and the feasibility of reuse. However, if the balance of the chemical ingredients are changed, the necessity of restoring the balance may be a limiting factor in its reuse.
The cleaning chemical sometimes requires treatment before discharge, such as pH adjustment (most often the pH is too high), toxic metal removal or other conditions. However, sometimes a discharge is not possible or undesirable, making hauling necessary.
Evaporative processes can also prove to be cost-effective. Since the rinse water contains an extremely low level of cleaning chemicals, it will typically meet the local discharge regulations without treatment. However, in some applications it is desirable to reduce and even eliminate rinse water discharge. The possible reasons may include cost effectiveness of recovering high purity rinse water, restrictions of water usage or a local ordinance preventing all discharge. There are two general methods used to accomplish this purpose. The ability to recycle wastewater rinses depends continuously on the characteristics of the contaminants. For aqueous, and, to a much lesser extent solvent cleaning, deionization is the key process. The ion-exchange resin continuously removes the process contamination and tap water, if tap water is used as makeup water to compensate for process water losses. If the contaminants are not removed continuously, they accumulate in the system and possibly redeposit on the parts being cleaned.
One or more of the following complementary processes such as mechanical filtration, activated carbon and membrane (reverse osmosis, ultrafiltration or microfiltration) are needed. Depending on the process requirement, mechanical filtration removes particles one micrometer and above. Activated carbon removes low levels of organic compounds (solvents, surfactants and others). Membranes can remove a large variety of particles such as dissolved minerals down to the ionic range. For reverse osmosis membranes, the wastewater is converted to water with purity ranging from 25,000 ohm-cm to 800,000 ohm-cm. If higher water purity is required, deionization can polish the wastewater up to 16.0 megohm-cm and higher. Ultrafiltration membranes are good for separating organic molecules from a wastewater stream. Typically, it is used to remove low molecular weight organic molecules (depending on the membrane molecular weight rating) such as oils or breaking oily emulsions. Microfiltration is primarily used to remove fine particles and breaking oily emulsions, but it can not remove the low molecular weight organic molecules that the ultrrafilter can. Also, neither ultrafilter nor microfilter membranes remove ionic species that the reverse osmosis membrane removes. Even though both membrane and microfiltration membranes are used to recycle cleaning chemicals, they can pretreat a wastewater (oily type) used in a closed-loop process prior to other purification methods such as reverse osmosis membrane or ion-exchange resin. The reject stream from any of these membranes is either reduced in volume by evaporation or hauled to a treatment facility.
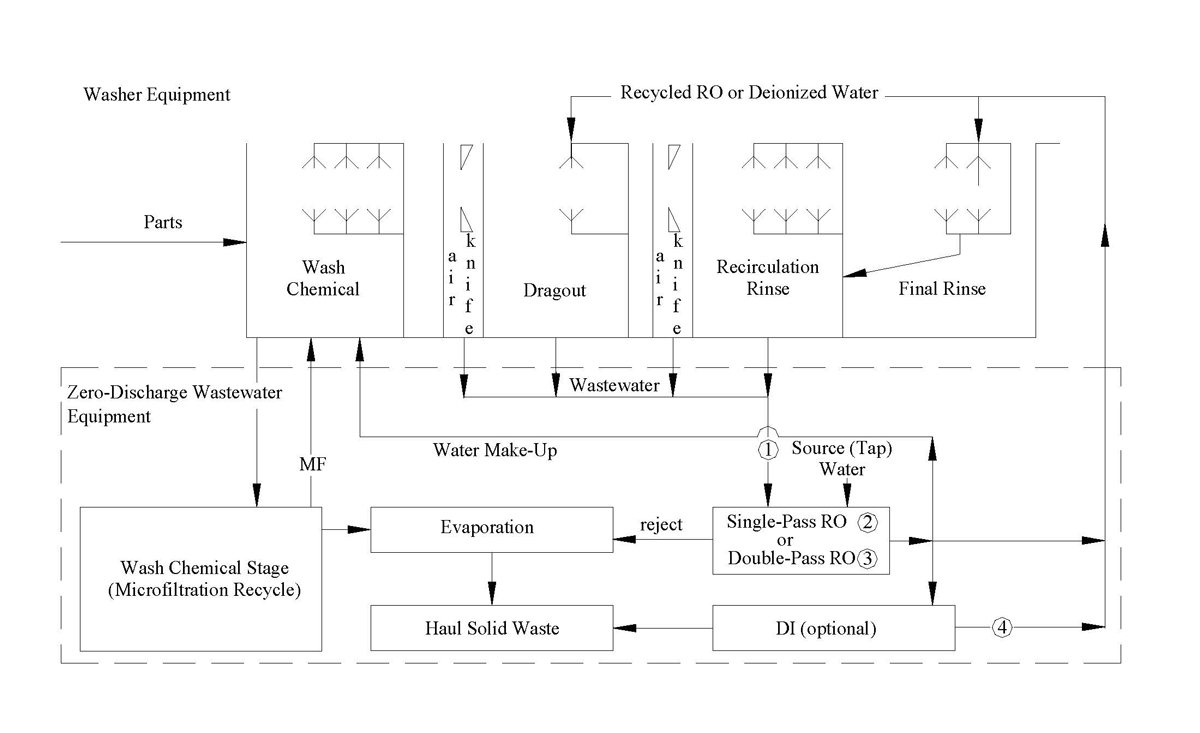
(Click Image to View Larger Version)
This schematic shows an alkaline wastewater treatment system using three separation technologies: evaporative, membrane (reverse osmosis) and ion exchange. The final rinse water component uses a closed-loop ion-exchange system. The membrane issued it separate the high ionic contamination from the dragout section into two streams, a reusable low purity water as makeup to the closed-loop system and a reusable alkaline cleaning chemical stream. Without the reverse osmosis membrane treating the wastewater from the dragout section of the cleaning equipment, the very high ionic content of the wastewater makes the final rinse water-recycling unit, which uses ion-exchange resin too expensive to operate. The evaporator further reduces the amount of spent cleaning chemical and the overflow from the wash tank caused by the excessive reject from the membrane. Other system designs can use one or more of the separation technologies depending on the application. For low volume batch operations an evaporator or just a closed-loop component may be used. If the discharge regulations are not a concern, then the membrane alone can be used to recycle the high water purity water while the reject stream can be discharged to the drain, if it meets the regulations.
Once you have minimized the amount of wastewater generated by the cleaning process, the solid waste disposal needs to be considered. Alkaline cleaning baths or spent solvents are solid wastes, even though they are actually liquids and sludges, per the federal EPA definition. As already mentioned, dewatering processes such as evaporation can be very effective in reducing disposal costs especially for hazardous wastes. Disposal costs may be as high as $5000.00 per 55-gallon drum. Other solid wastes, such as filters will have to be disposed of as they are or even incinerated depending on the contamination that may cause a regulated emission. Reducing the amount of this non-liquid and sludge solid waste depends on the selection of filters. The best selections are those filters with the highest removal of contaminants at the lowest cost. For the ion-exchange resins, reuse is possible depending on numerous factors such as the water purity desired, type of contaminants being removed and shipping costs.
The selection of separation technologies and system designs can be an important strategy in avoiding the classification of some solid wastes as hazardous. The Federal EPA standard for classification of solid wastes, the "TCLP" test, is used with few exceptions by most states to classify wastes as hazardous. It is possible to avoid the hazardous waste classification by knowing the test criteria and the peculiarities of the test method.
Summary
The minimization of the amount of wastewater generated by a cleaning process depends on numerous factors. By simultaneously evaluating the entire cleaning process by cleaning chemistry, cleaning equipment and wastewater treatment equipment, you will achieve your objective with minimal capital and operating costs.
Compatibility of Temporary Water Soluble Spot Mask and Defoamer Soldering Chemistries in a Closed-Loop Water Recycling System
Temporary Water Soluble Mask
Case History - A closed-loop wastewater recycling user was dissatisfied with the spot mask that he was using because it was leaving a residue inside the cleaner. The accumulated residue inside the windows left doubts about the cleanliness of the printed circuit boards.
Solution - After testing several types of mask he found one that performed best for his application. The use of this mask produced an immediate positive effect - there was no residue accumulation! As he continued to use the mask other benefits became apparent such as reduced pump repairs, frequency of cleaning the cleaner and, most importantly, the achievement of a substantially longer media life. After comparing the operating costs, he found that they were reduced from $10,000 per year to $7,000 per year. Since this experience, SepTech has used this specific mask and has found that it will always reduce the operating costs of the recycle system in comparison to other brands of spot masks.
Defoamer
Case History - A new AquaCycler was started-up and the water purity varied from 10 to 18 megohm-cm for a few days. On one occasion the water purity suddenly dropped to 450,000 ohm-cm but the water regained its higher purity level over the next few days. However, on another occasion, after adding the defoamer, the engineer noticed that the addition of 2 capfuls of defoamer caused the water purity dropped from about 10 megohm-cm down to 150,000 ohm-cm in minutes! This amount of defoamer was necessary to reduce the foam level before it overflowed from the cleaner wash tank.
Solution - The water recycling system could now operate without being prohibitively expensive. In addition, there was no visible decrease in the water purity and only 5 drops were needed to achieve the same result as two capfuls of the other brand!

